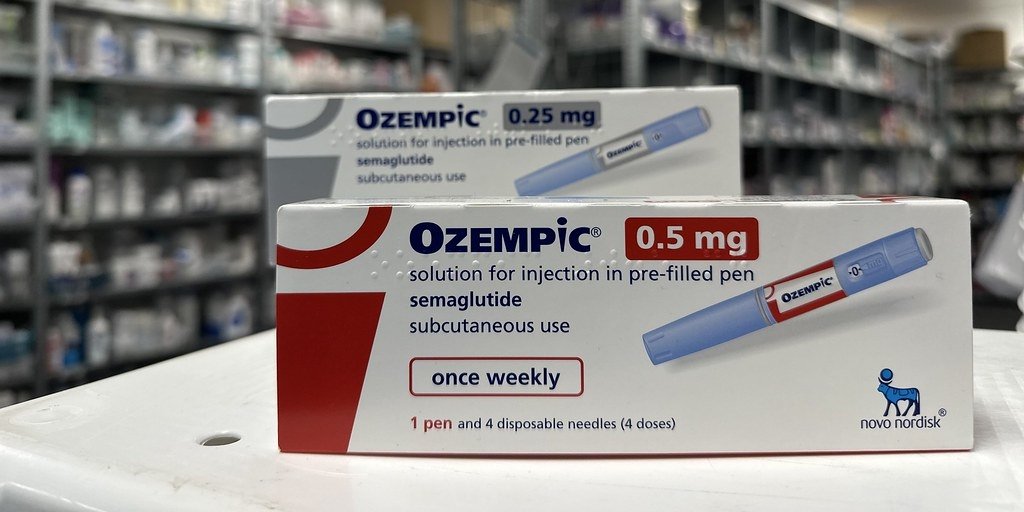
The US Food and Drug Administration (FDA) has issued a warning regarding the discovery of counterfeit Ozempic (semaglutide) injections within the American drug supply chain. This alert is directed at consumers, patients, healthcare professionals, and pharmacies.
On April 3, Novo Nordisk, the producer of Ozempic, informed the FDA that numerous fake products had been circulated outside their authorized supply channels. Following this, the FDA seized these counterfeit items on April 9 and initiated an investigation to determine the extent of the issue.
While Novo Nordisk mentioned six adverse events linked to the affected batch, these do not seem to be connected to the counterfeit products. Both the FDA and Novo Nordisk are currently analyzing the seized items to assess their identity, quality, and safety. The investigation is still ongoing.
Ozempic is classified as a glucagon-like peptide 1 (GLP-1) receptor agonist, utilized alongside diet and exercise to enhance glycemic control in adults with type 2 diabetes. It’s also recognized for diminishing the chance of significant cardiovascular incidents like heart attacks and strokes in adults suffering from type 2 diabetes and existing cardiovascular conditions.
In response, the FDA has provided guidance aimed at safeguarding public health.
Patients, including wholesalers and retail pharmacies, are advised to check the lot number PAR0362 and the serial number for Ozempic products beginning with 51746517. Products matching these identifiers should not be used, sold, or distributed.
Retail pharmacies suggest acquiring Ozempic solely through accredited Novo Nordisk distributors and to thoroughly inspect the packaging for signs of authenticity.
Patients should ensure they obtain Ozempic from a duly licensed pharmacy with a legitimate prescription and should check the product for any signs of counterfeiting prior to use.
If individuals suspect they may have received counterfeit or tampered medication, they should report it through the FDA’s MedWatch System.
Retailers and consumers with any questions or concerns are encouraged to reach out to Novo Nordisk’s Customer Care at 1-800-727-6500.
For further information, the FDA recommends consulting the official announcement from Novo Nordisk.